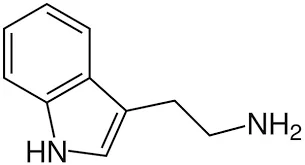
Tryptamine is an indole-based amine with the molecular formula C10H12N2. It contains an indole ring connected to an ethylamine side chain, which confers both biological activity and synthetic versatility.
1. Molecular Formula: C10H12N2
2. CAS Number: 61-54-1
3. Appearance: White to off-white crystalline powder
4. Purity: 99% (suitable for research and synthesis)
5. Solubility: Soluble in ethanol, methanol, chloroform, and partially soluble in water
Its structure enables Tryptamine to act as a precursor to various neuroactive compounds and psychoactive derivatives, making it invaluable in medicinal chemistry and neuroscience research.
Tryptamine has wide-ranging applications in research, pharmaceutical synthesis, and biological studies:
1. Neurotransmitter Precursor: Tryptamine is a natural precursor to serotonin, melatonin, and other indolealkylamine neurotransmitters, making it essential for studies in neuroscience, sleep regulation, and mood disorders.
2. Pharmacology and Medicinal Chemistry: Tryptamine serves as a building block in the synthesis of psychoactive compounds, triptans (used for migraine treatment), and other indole derivatives, allowing researchers to explore novel therapeutic agents.
3. Chemical Synthesis: Its amine functionality and indole ring make Tryptamine versatile for alkylation, acylation, and condensation reactions, serving as a starting point for synthesizing complex organic molecules.
4. Research Tool: Widely used in laboratory research, Tryptamine is utilized to study receptor binding, enzyme activity, and the biosynthesis of neurotransmitters, offering valuable insights into brain chemistry and metabolic pathways.
Tryptamine should be handled with care in a controlled laboratory environment:
1. Protective Equipment: Use gloves, safety goggles, and lab coats when handling the compound.
2. Ventilation: Work in a well-ventilated fume hood to avoid inhalation of dust or vapors.
3. Storage: Store in a cool, dry place, away from light, heat, and moisture. Keep containers tightly sealed.
4. Disposal: Dispose of in accordance with local regulations for chemical waste, following institutional guidelines.
With the deepening implementation of the "Made in China 2025" strategy and the rapid advancement of high-end equipment manufacturing, China's coupling industry is accelerating its transformation toward intelligent and precision-oriented development.

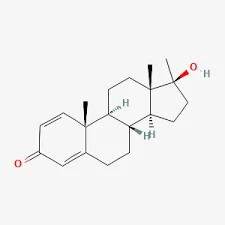
The global conversation around performance-enhancing drugs has intensified once again as anabolic steroids, particularly products marketed as Oil Dianabol, continue to surface in illegal markets. Dianabol, scientifically known as methandrostenolone, is one of the most well-known anabolic steroids in bodybuilding history. Originally developed in the mid-20th century for medical use, it has long since become controversial due to widespread misuse, health risks, and regulatory crackdowns.